The full company announcement is available here.
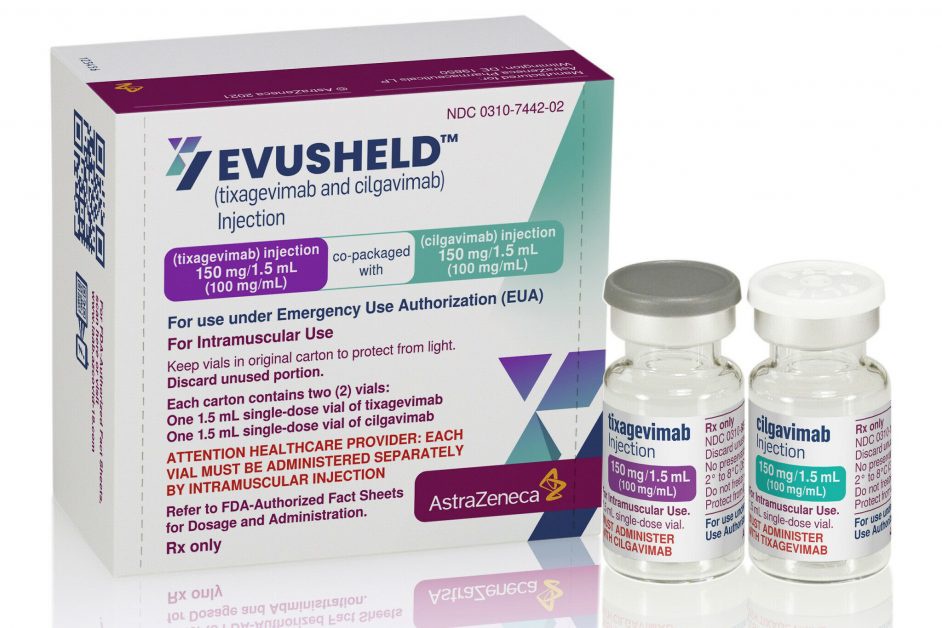
The approval by the European Commission was based on results from the Evusheld clinical development programme, including data from the PROVENT Phase III pre-exposure prophylaxis trial which showed a 77% reduction in the risk of developing symptomatic COVID-19 compared to placebo at the primary analysis and an 83% reduction at a six-month median analysis, with protection from the virus lasting at least six months.1-3 Evusheld was generally well-tolerated in the trial.1-3
James Teague, Country President, AstraZeneca (Thailand) Ltd., said "As Thailand transitions from a pandemic to an endemic stage, we are committed to leave no patient behind. Evusheld is an option to provide additional protection to immunocompromised people, particularly the 607 population who are most vulnerable to the virus. They benefit from the pre-exposure prophylaxis with Evusheld.This EMA approval is really good news, and we are doing our best to make it available in Thailand soon."
Evusheld is authorised for emergency use for pre-exposure prophylaxis of COVID-19 in the US and has been granted conditional marketing authorisation by the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain for pre-exposure prophylaxis of COVID-19. Additionally, there are a number of countries across Europe that have agreements in place to provide Evusheld.
AstraZeneca
AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide.
AstraZeneca is based in six different locations across the UK, with its global headquarters in Cambridge. In the UK, almost 8,000 employees work in research and development, manufacturing, supply, sales and marketing. We supply 34 different medicines to the NHS, which treat more than one million UK patients every year. For more information, please visit www.astrazeneca.co.uk and follow us on Twitter @AstraZenecaUK.
Source: AstraZeneca